近日,林永教授团队在《 CELL DISCOVERY 》杂志上发表“The SARS-CoV-2 protein ORF3a inhibits fusion of autophagosomes with lysosomes”的论文。
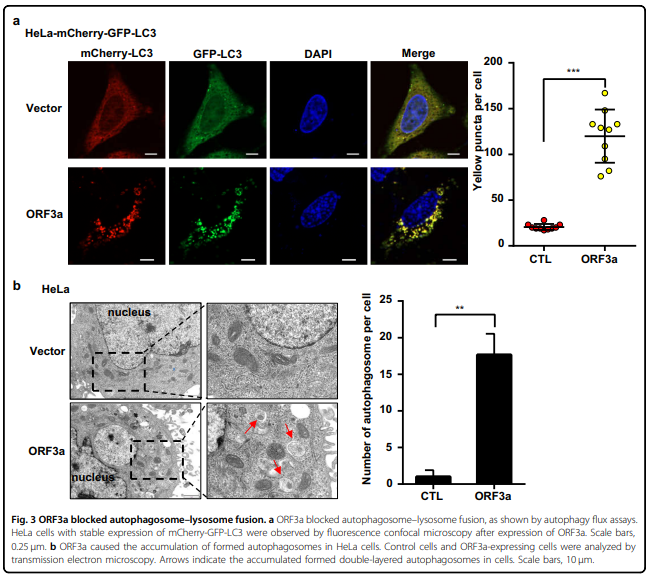
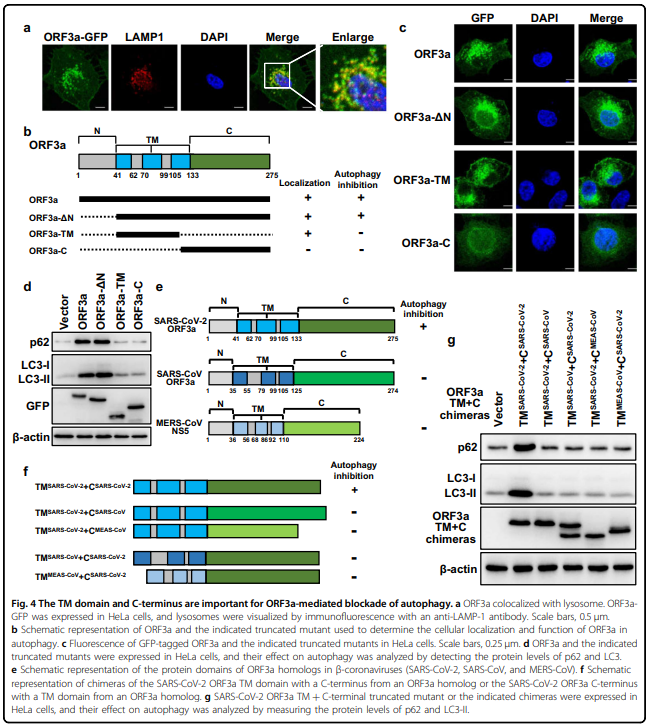
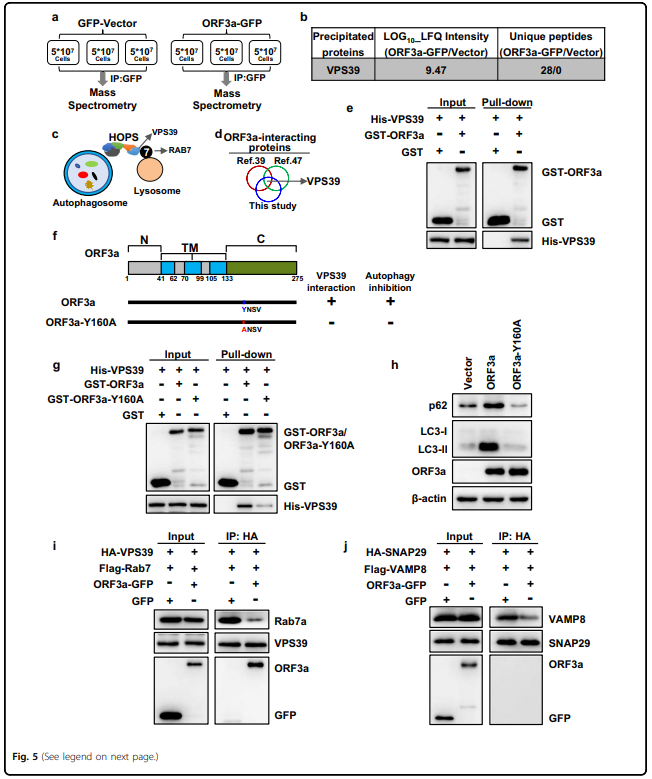
严重急性呼吸系统综合征冠状病毒2 (SARS-CoV-2)引发了2019年冠状病毒病大流行。 SARS-CoV-2如何调节细胞反应以逃避宿主细胞的清除尚不清楚。 自噬是一种胞内溶酶体降解途径,用于清除各种货物,包括病毒。 在这里,我们系统筛选了SARS-CoV-2的28个病毒蛋白,发现ORF3a通过阻断自噬体与溶酶体的融合,强烈抑制了自噬通量。 ORF3a与溶酶体共定位,与VPS39相互作用, 同型融合和蛋白质分拣(HOPS)复合体的一个组成部分。 ORF3a-VPS39相互作用阻止了HOPS与RAB7的结合,从而阻止了融合机制的组装,导致未融合自噬体的积累。 这些结果揭示了SARS-CoV-2逃脱降解的潜在机制; 也就是说,病毒干扰自噬体-溶酶体融合。
该发现将促进针对自噬的策略,以提供潜在的保护,以防止SARS-CoV-2的传播。
The SARS-CoV-2 protein ORF3a inhibits fusion of
autophagosomes with lysosomes
Yabin Zhang , Hao Sun , Rongjuan Pei , Binli Mao , Zhenyu Zhao , Huihui Li , Yong Lin and Kefeng Lu
Zhang et al. Cell Discovery (2021) 7:31
https://doi.org/10.1038/s41421-021-00268-z
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused the ongoing coronavirus disease 2019
pandemic. How SARS-CoV-2 regulates cellular responses to escape clearance by host cells is unknown. Autophagy is
an intracellular lysosomal degradation pathway for the clearance of various cargoes, including viruses. Here, we
systematically screened 28 viral proteins of SARS-CoV-2 and identified that ORF3a strongly inhibited autophagic flux by
blocking the fusion of autophagosomes with lysosomes. ORF3a colocalized with lysosomes and interacted with VPS39,
a component of the homotypic fusion and protein sorting (HOPS) complex. The ORF3a–VPS39 interaction prohibited
the binding of HOPS with RAB7, which prevented the assembly of fusion machinery, leading to the accumulation of
unfused autophagosomes. These results indicated the potential mechanism by which SARS-CoV-2 escapes
degradation; that is, the virus interferes with autophagosome–lysosome fusion. Furthermore, our findings will facilitate
strategies targeting autophagy for conferring potential protection against the spread of SARS-CoV-2.